Chronic Lymphocytic Leukemia (CLL) is an indolent lymphoproliferative disorder commonly managed in community centers. Ibrutinib revolutionized the management of patients with CLL (2). Ibrutinib was initially considered an easy to administer, well tolerated oral medication with excellent efficacy. It was the first clinically available Bruton's Tyrosine Kinase (BTK) inhibitor widely used to treat CLL (3). Recently there have been concerns about cardiovascular toxicities leading to the use of alternative BTK inhibitors (5). Where alternative BTK inhibitors are available these toxicity concerns have led to a decline in the use of ibrutinib to treat CLL.
The population served is ethnically diverse consisting of 52% South East Asian with a significant population of African origin.
We suspected that the concerns regarding Ibrutinib toxicity were not seen in our patient population prompting a retrospective review of our experience.
Ibrutinib therapy
A total of 106 CLL patients treated with Ibrutinib were identified from which data was extracted. The mean age was 63.4 years. Patients with CLL treated with Ibrutinib from 2014 to 2021 were identified using OPIS and Meditech and data was extracted manually.
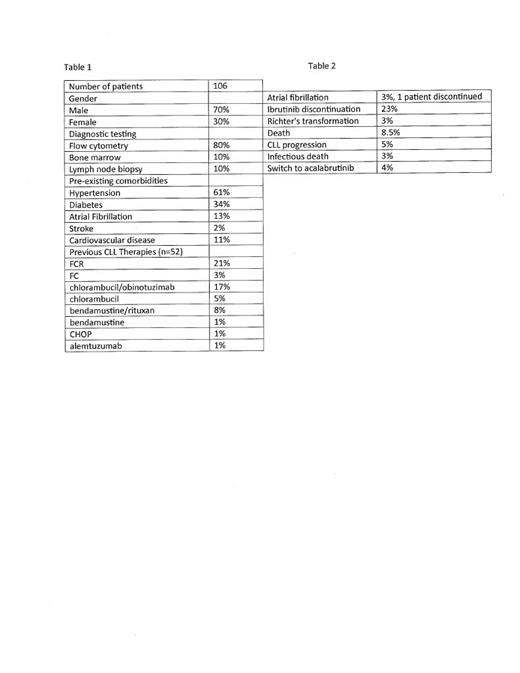
Table 1
60% of patient received their initial drug funding through compassionate access, 2% via private insurance and the remaining 38% through the provincial health system. The majority of patients who received funding through compassionate access were eventually transitioned to the provincial health system for drug funding.
Toxicity and outcomes
Table 2
81% of treated patients never required a dose adjustment of Ibrutinib. The majority of dose reductions were of a temporary nature.
We were unable to obtain accurate response data in our retrospective review. 82 of the 106 patients continue on ibrutinib therapy. It is presumed that these patients continue to benefit from therapy without disease progression although response data details were poorly recorded in the medical record.
Conclusions
There is a lack of data of community real world experience with Ibrutinib particularly in the Canadian setting. Community centers generally are lacking in information technology required to do retrospective audits.
Our data shows that ibrutinib therapy for CLL is a reasonable and effective option with good tolerability in a patient population with high cardiovascular risk. The data is limited by the retrospective data collection.
The efficacy of therapy is likely comparable to that of the published literature.
These data suggest that the tolerability of Ibrutinib therapy seems better than that of the published literature (1) with a low incidence of atrial fibrillation, dose adjustments and low drug discontinuation rate. Of note Richter's transformation was also uncommon in our patient population. This is particularly of interest as our study did capture patients in the early days of ibrutinib therapy when there were concerns that Richter's transformation was more common than seen today.
Many of the patients who were treated with ibrutinib would not have received the drug today because of cardiovascular concerns (4). At that time, it was the only available oral targeted agent for the treatment of chronic lymphocytic leukemia. Other agents such as Venetoclax, Acalabrutinib, Zanubrutinib and novel combinations were unavailable at that time or were not funded. These lack of options likely contribute to the persistence of Ibrutinib therapy in our patients.
These data suggest that ibrutinib can be used in a high cardiovascular risk population with good efficacy and tolerability.
1.Alloucjery, M., Tomowiak, C., Lombard, T., & et al. (2021). Safety Profile of Ibrutinib: An Analysis of the WHO Pharmacovigilance Database. Front Pharmacology, 12:769315.
2.Byrd, J. C., Brown, J. R., O'Brien, S., & et al. (2014). Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. NEJM, 371(3):213-223.
3.Byrd, J. C., Furman, R. R., & Coutre, S. S. (2013). Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. NEJM, 369(1):32-42.
4.Lovell, A. R., Jammal, N., & Bose, P. (2022). Selecting the optimal BTK inhibitor therapy in CLL: rationale and practical considerations. Therapueltic Advances in Hematology, 13:1177/20406207221116577.
5.Salem, J. E., Manouchehri, A., & Bretagne, M. (2019). Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. J Am Coll Cardiol, 74:1667-1678.
Disclosures
Kuruvilla:Janssen, AstraZeneca: Honoraria; Abbvie, Novartis, Sanofi: Other: Advisory Board; Janssen: Other: TAILOR Steering Committee.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal